| Identification of Cry receptors in Lepidoptera | |||
Which are the functional Cry insecticidal protein receptors?Cadherins, aminopeptidases, glycolipids, alkaline phosphatases, and ATP binding cassette (ABC) transporters have all been proposed as functional receptors for Cry proteins from the bacterium Bacillus thuringiensis in Lepidoptera. The goal of our project is to identify and characterize Cry toxin binding proteins and test their role as functional Cry toxin receptors. As insect models we use diverse lepidopteran species that are relevant agricultural pests, including Heliothis virescens (tobacco budworm), Helicoverpa zea (cotton bollworm), Spodoptera frugiperda (fall armyworm), Chrysodeixis includens (soybean looper) and Anticarsia gematalis (velvetbean caterpillar). We have also used alternative insect pests as they become available or are of interest to our group or collaborators. Because of their use in transgenic Bt crops, we have mostly focused on identifying receptors for Cry1Ac, Cry1Fa, and Cry2Ab toxins. More recently we have expanded our efforts to novel Cry proteins with potential use in new transgenic crops or Bt pesticides, such as Cry1I or Cry9 proteins. We use diverse techniques to identify receptors, including but not limited to binding competition assays with radiolabeled Cry proteins, proteomics and blotting, affinity chromatography, and cloning followed by heterologous expression and cytotoxicity assays testing the fucntion of candidate Cry protein receptor genes. Identification of these receptors is crucial in identifying cros-resistance risks and designing monitoring as well as resistance management strategies. 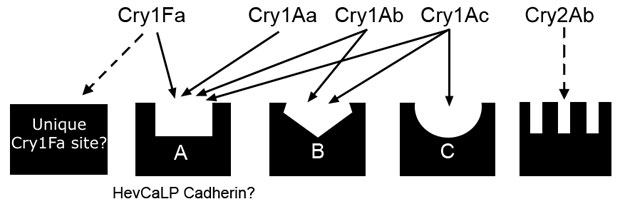 Current Cry toxin binding site model for H. virescens larvae from binding competition assays. Predicted binding to unidentified sites is displayed with a broken line. These models are used to identify Cry proteins that do not share target sites in the insect midgut so that they can safely be combined in Bt crops or pesticides to delay evolution of resistance. |
|||
Selected publications from this area of research |
Mushtaq, R., R. Behle, R. Liu, L. Niu, P. Song, A. R. Shakoori, and J. L. Jurat-Fuentes (2017) "Activity of Bacillus thuringiensis Cry1Ie2, Cry2Ac7, Vip3Aa11 and Cry7Ab3 proteins against Anticarsia gemmatalis, Chrysodeixis includens and Ceratoma trifurcata" J. Invertebr. Pathol. 150: 70-72. PDF |
Jurat-Fuentes, J. L., and N. Crickmore (2017) "Specificity determinants for Cry insecticidal proteins: Insights from their mode of action" J. Invertbr. Pathol. 142: 5-10. PDF |
Perera, O. P., Shelby, K. S., Popham, H. J. R., Gould, F., Adang, M. J., and J. L. Jurat-Fuentes (2015) "Generation of a transcriptome in a model lepidopteran pest, Heliothis virescens, using multiple sequencing strategies for profiling midgut gene expression" PLoS ONE 10(6): e0128563. PDF |
Gong, L., Wang, H., Qi, J., Han, L., Hu, M., and J. L. Jurat-Fuentes (2015) "Homologs of Cry toxin receptor genes in a de novo transcriptome and their altered expression in resistant Spodoptera litura larvae" J. Invertebr. Pathol. 129: 1-6. PDF |
Zhao, C., Jurat-Fuentes, J. L., Abdelgafar, H. M., Pan, H., Song, F., and J. Zhang (2015) "Identification of a new cryI-type gene as candidate for gene pyramiding in corn to control Ostrinia spp. larvae” Appl. Environ. Microbiol. 8(11): 3699-3705. PDF |
Han, L., Han, C., Liu, Z., Chen, F., Jurat-Fuentes, J. L., Hou, M., and Y. Peng (2014) "Binding site concentration explains the differential susceptibility of Chilo suppresalis and Sesamia inferens to Cry1A-producing rice” Appl. Environ. Microbiol. 80(16): 5134-5140. PDF |
Tanaka, S., Miyamoto, K., Noda, H., Jurat-Fuentes, J. L., Endo, H., and R. Sato (2013) "The ATP-binding cassette transporter subfamily C member 2 in Bombyx mori larvae is a functional receptor for Cry toxins from Bacillus thuiringiensis” FEBS J. 280(8): 1782-1794. PDF |
Gouffon, C., Van Vliet, A., Van Rie, J., Jansens, S., and J. L. Jurat-Fuentes (2011) “Binding sites for Cry2Ae toxin from Bacillus thuringiensis on heliothine brush border membrane vesicles are not shared with Cry1A, Cry1F or Vip3A toxins” Appl. Environ. Microbiol. 77(10): 3182-3188. PDF |
Ning, C., Wu, K., Liu, C., Gao, Y., Jurat-Fuentes, J.L., and X. Gao (2010) "Characterization of a Cry1Ac toxin-binding alkaline phosphatase in the midgut from Helicoverpa armigera (Hubner) larvae" J. Insect Physiol. 56(6):666-672. PDF |
Perera, O.P., Willis, J.D., Adang, M.J., and J.L. Jurat-Fuentes (2009) “Cloning and characterization of the Cry1Ac-binding alkaline phosphatase (HvALP) from Heliothis virescens” Insect Biochem. Molec. Biol. 39: 294-302. PDF |
Jurat-Fuentes, JL and Adang, MJ (2006) The Heliothis virescens cadherin protein expressed in Drosophila S2 cells functions as a receptor for Bacillus thuringiensis Cry1A but not Cry1Fa toxins. Biochemistry 45: 9688-9695. PDF |
Jurat-Fuentes, JL, Gahan, LJ, Gould, FL, Heckel, DG and Adang, MJ (2004) The HevCaLP protein mediates binding specificity of the Cry1A class of Bacillus thuringiensis toxins in Heliothis virescens. Biochemistry 43: 14299-14305. PDF |
Jurat-Fuentes, JL and Adang, MJ (2001) Importance of Cry1 delta-endotoxin domain II loops for binding specificity in Heliothis virescens (L.). Appl. Environ. Microbiol. 67: 323-329. PDF |
Department of Entomology and Plant Pathology
The University of Tennessee
370 Plant Biotechnology Building
2505 E. J. Chapman Drive
Knoxville, TN, 37996
Tel: (865) 974-5931
jurat@utk.edu
